Abstract
Introduction
Fms-like tyrosine kinase 3 internal tandem duplications (FLT3/ITD) are among the most common mutations found in AML patients. Interestingly, although initial remission rates of FLT3/ITD AML are similar to standard-risk AMLs, these AML have a high relapse rate and poor overall survival. Moreover, FLT3 TKIs have been much more successful in eliminating peripheral FLT/ITD blasts, than eradicating marrow disease and generating durable responses clinically. Studies have addressed several mechanisms underlying the FLT3 TKI resistance, including a protective role for the bone marrow (BM) microenvironment. However, the mechanisms underlying this protection remain unknown. We previously showed that human bone marrow stromal cells (BMSCs) highly express most cytochrome P450 (CYP) enzymes, and these enzymes are a major means of chemoprotection within the BM niche (Alonso et al. Oncotarget 2015). Among them, CYP3A4 is a key enzyme responsible for hepatic inactivation of many chemotherapy agents, including many FLT3 TKIs used clinically. Therefore, we hypothesized that BM stromal CYP3A4 contributes to BM microenvironment mediated FLT3 TKI resistance by FLT3/ITD AML.
Methods and Results
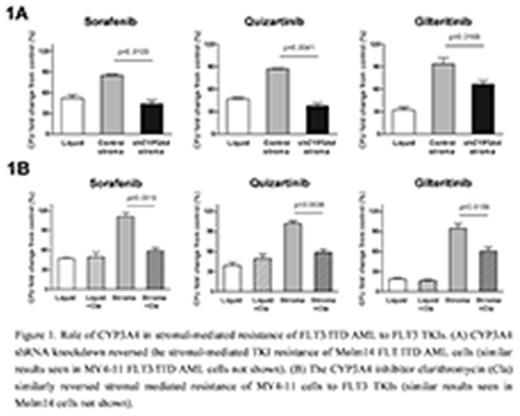
To test our hypothesis in vitro, FLT3/ITD AML cell lines (MV4-11 and Molm14) were co-cultured with FLT3 TKIs with or without BMSCs (F/STRO), and their clonogenic activity was assessed. We found that 3 different clinically active FLT3 TKIs (sorafenib, quizartinib, and gilteritinib), while active against the FLT3/ITD AML in liquid culture, had little activity when co-cultured with BMSCs (Figure 1A). Moreover, when FLT3/ITD AMLs were co-cultured with BMSCs with short hairpin (sh) RNA knockdown of CYP3A4 (shCYP3A4), the activity of the FLT3 TKIs was restored (Figure 1A). To test the hypothesis in vivo, we investigated the growth of FLT3/ITD AML tumors containing control or shCYP3A4 human primary BMSCs upon FLT3 TKI treatments in a xenograft mouse model. We observed that the tumors with shCYP3A4 BMSCs were significantly more sensitive to FLT3 TKI treatments compared to those with control BMSCs.
To further examine the role of CYP3A4 in BMSCs, we examined the inhibitory effects of FLT3 TKIs in the absence of stromal cellular contact with FLT3/ITD AML cells. FLT3 TKI-containing media was incubated with and without control BMSCs and shCYP3A4 BMSCs, and then incubated with FLT3/ITD AML cells. Western blot performed on the FLT3/ITD AML cells showed that FLT3 TKI media conditioned with BMSCs blocked the inhibition of FLT3 phosphorylation, and this was reversed by CYP3A4 knockdown in the BMSCs. These results demonstrated that stromal CYP3A4 protected against FLT3 TKIs even in the absence of stromal cell contact.
We next studied if combining a clinically active CYP3A4 inhibitor with FLT3 TKIs would increase the FLT3/ITD AML cells' sensitivities to FLT3 TKIs in the presence of BMSCs. Clarithromycin, a clinically active CYP3A4 inhibitor, significantly reversed the protective effects of BMSCs to 3 different FLT3 TKIs (Figure 1B).
Conclusions
These data confirm the protective effects of stroma for FLT3/ITD AML against FLT3 TKIs. Further, we found for the first time that CYP3A4 expressed in BMSCs contributes to BMSC mediated FLT3/ITD AML protection against FLT3 TKIs both in vitro and in vivo mouse model. Our results also demonstrated that combining FLT3 TKIs with clinically available CYP3A4 inhibitors may become a promising strategy to overcome drug resistance in FLT3/ITD AML treatment.
Levis: Novartis: Consultancy, Honoraria, Research Funding; Daiichi Sankyo, Inc.: Consultancy, Honoraria; Astellas Pharma Us: Consultancy, Research Funding; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding; FujiFilm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal